|
28.03.2017 15:15:00
|
Medical Marijuana, Inc. Major Investment AXIM Biotech Enters Term Sheet Agreement With U.S. API Company To Develop Bioequivalent Product To Marinol
SAN DIEGO, March 28, 2017 /PRNewswire/ -- Medical Marijuana, Inc. (OTC: MJNA), the first-ever publicly traded cannabis company in the United States, announced today that its major investment company AXIM® Biotechnologies, Inc. (AXIM® Biotech) (OTCQB: AXIM), has entered into a Term Sheet Agreement with a U.S.-based controlled-substances API (Active Pharmaceutical Ingredient) production company to develop a dronabinol-based functional, controlled-release chewing gum product based on AXIM IP and technology. The new dronabinol chewing gum product will be bioequivalent to Marinol®. Marinol® was first introduced in the U.S. market in 1985 and remains the only FDA approved cannabis-based drug available in the U.S.
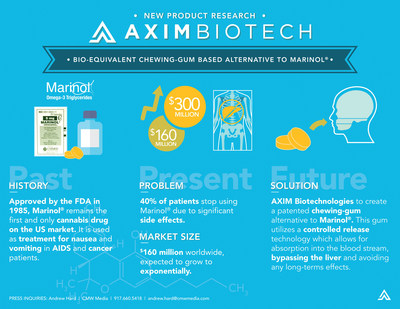
Marinol (generic name: Dronabinol) is used to treat nausea and vomiting caused by cancer chemotherapy. It is usually used when other drugs to control nausea and vomiting have not been successful. Dronabinol is also used to treat loss of appetite and weight loss in patients with HIV infection. Dronabinol (also called THC) is a man-made form of the active natural substance in marijuana. AXIM will pursue these same indications in its clinical research on the chewing gum replacement.
In its current form, Marinol is delivered through a gel capsule where 90% of the dronabinol is metabolized into 11-OH- THC due to the first-pass (liver) metabolism. This causes significant side effects for patients including impaired thinking and other adverse reactions. AXIM's patented control-release chewing gum largely bypasses the first pass metabolism in the liver to the point where AXIM believes their new formulation will improve efficacy while simultaneously reducing side effects for patients who utilize this new delivery method.
AXIM will work with an API-manufacturing company that will supply AXIM with the synthetic dronabinol (delta-9-THC) for use in these trials. AXIM will then conduct a bio-equivalency study and will eventually seek FDA approval to bring this chewing gum product to market.
"AXIM has once again expanded its clinical research program in an impressive and important way that will greatly benefit this population of patients," said Dr. Stuart Titus, PhD, CEO of Medical Marijuana, Inc. "With more than a third of patients discontinuing use of Marinol® as prescribed due to adverse side effects, it is encouraging that AXIM will research and attempt to bring to market an improved medication that may provide expanded therapeutics while allowing for a better quality of life for this patient population."
About AXIM®
AXIM® Biotechnologies, Inc. (OTC: AXIM) focuses on the research, development and production of cannabis-based pharmaceutical, nutraceutical and cosmetic products. Our flagship products include CanChew®, a CBD-based controlled release chewing gum, and MedChew Rx, a combination CBD/THC gum that is undergoing clinical trials for the treatment of pain and spasticity associated with multiple sclerosis. We prioritize the well-being of our customers while embracing a solid fiscal strategy. For more information, please visit www.AXIMBiotech.com.
About Medical Marijuana, Inc.
Our mission is to be the premier cannabis and hemp industry innovators, leveraging our team of professionals to source, evaluate and purchase value-added companies and products, while allowing them to keep their integrity and entrepreneurial spirit. We strive to create awareness within our industry, develop environmentally-friendly, economically sustainable businesses, while increasing shareholder value. For details on Medical Marijuana, Inc.'s portfolio and investment companies, visit www.medicalmarijuanainc.com.
To see Medical Marijuana, Inc.'s video statement, click here.
Shareholders are also encouraged to visit the Medical Marijuana, Inc. Shop for discounted products.
FORWARD-LOOKING DISCLAIMER AND DISCLOSURES This press release may contain certain forward-looking statements and information, as defined within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, and is subject to the Safe Harbor created by those sections. This material contains statements about expected future events and/or financial results that are forward-looking in nature and subject to risks and uncertainties. Such forward-looking statements by definition involve risks, uncertainties.
The statements in this press release have not been evaluated by the FDA and are not intended to diagnose, treat or cure any disease. The Company does not sell or distribute any products that are in violation of the United States Controlled Substances Act. The Company does sell and distribute hemp-based products.
PUBLIC RELATIONS CONTACT:
Andrew Hard
Chief Executive Officer
CMW Media
P: 888-829- 0070
andrew.hard@cmwmedia.com
www.cmwmedia.com
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/medical-marijuana-inc-major-investment-axim-biotech-enters-term-sheet-agreement-with-us-api-company-to-develop-bioequivalent-product-to-marinol-300430175.html
SOURCE Medical Marijuana, Inc.
 Der finanzen.at Ratgeber für Aktien!
Der finanzen.at Ratgeber für Aktien!
Wenn Sie mehr über das Thema Aktien erfahren wollen, finden Sie in unserem Ratgeber viele interessante Artikel dazu!
Jetzt informieren!
Nachrichten zu Medical Marijuana Inc.mehr Nachrichten
| Keine Nachrichten verfügbar. |
Analysen zu Medical Marijuana Inc.mehr Analysen
Aktien in diesem Artikel
| AXIM Biotechnologies Inc | 0,00 | -4,55% |
|
| Medical Marijuana Inc. | 0,00 | 14,29% |
|