|
05.12.2022 17:00:00
|
Imagen Technologies Announces FDA Clearance of Aorta-CAD
New AI device expands Diagnostics as a Service platform capabilities.
NEW YORK , Dec. 5, 2022 /PRNewswire/ -- Imagen Technologies, Inc. announced the U.S. Food and Drug Administration's 510(k) clearance of the computer-assisted detection (CADe) device Aorta-CAD. This new, FDA cleared device is designed to assist physicians at detecting findings on chest X-rays that are suggestive of Aortic Atherosclerosis and Aortic Ectasia.
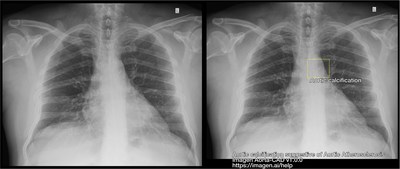
Designed to assist physicians detect findings on chest X-rays suggestive of Aortic Atherosclerosis and Aortic Ectasia.Aorta-CAD is a cloud-based, software-only medical device that uses deep learning to detect and highlight findings associated with Aortic Atherosclerosis and Aortic Ectasia. The device is part of Imagen's Diagnostics as a Service (DaaS) platform. It can assist any physician who reads chest X-rays, including radiologists and Primary Care Physicians (PCPs).
This FDA clearance, Imagen's 4th, marks another major milestone for the company as it seeks to improve patient outcomes by making frontline imaging diagnostics suitable for PCP offices. By adding the Aorta-CAD device to its DaaS platform, Imagen expects to help Primary Care Physicians detect and treat these chronic diseases earlier and better.
With assistance from Aorta-CAD, physicians are better equipped to diagnose and start treating Aortic Atherosclerosis and Aortic Ectasia earlier. Aorta-CAD presents an opportunity for practices to identify these previously undiagnosed chronic ailments, improve quality of life for their patients, and reduce total cost of care.
Aorta-CAD identifies findings on the chest X-ray image and creates annotations that draw attention to those findings via an overlay. The overlay can be toggled off when not being used by the physician. It seamlessly integrates into existing X-ray reading workflows without the need for new PACS technology or costly re-training of physicians.
"Imagen is on a mission to help Primary Care Physicians deliver faster, better diagnoses and care plans. By expanding our diagnostics as a service platform with Aorta-CAD, we expect to help PCPs significantly improve the quality of care and clinical outcomes for their patients with undiagnosed Aortic Atherosclerosis and Aortic Ectasia," says Alex Dresner, CEO at Imagen Technologies.
Like other components of Imagen's platform, Aorta-CAD delivers significant results for patients, physicians, and the health care system. Per Aorta-CAD's clinical trial:
- 62% relative reduction by physicians in misses for aortic calcification suggestive of Aortic Atherosclerosis
- 35% relative reduction in misses for dilated aorta suggestive of Aortic Ectasia
- 96% of physicians showed improvement when assisted by Imagen's device
"Aorta-CAD assists the physician to have a consistent and repeatable process to look into the cardiac silhouette providing additional information to enhance the patient's treatment plans and care," said Scott Howell, Imagen's Chief Medical Officer.
About Imagen Technologies, Inc. Imagen's mission is to improve the accessibility, accuracy, and immediacy of frontline diagnostic imaging. Our Diagnostics as a Service (DaaS) platform improves the patient experience and empowers primary care physicians to make better, earlier diagnoses so they can deliver higher-quality care. Imagen's DaaS platform seamlessly integrates imaging equipment, technical staff, radiologists, imaging software, and proprietary FDA cleared AI devices at practices big and small. It enables modern primary care practices to quickly bring frontline imaging diagnostics to the point of care, improving the patient experience, care quality, and practice economics.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/imagen-technologies-announces-fda-clearance-of-aorta-cad-301692229.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/imagen-technologies-announces-fda-clearance-of-aorta-cad-301692229.html
SOURCE Imagen Technologies
 Der finanzen.at Ratgeber für Aktien!
Der finanzen.at Ratgeber für Aktien!
Wenn Sie mehr über das Thema Aktien erfahren wollen, finden Sie in unserem Ratgeber viele interessante Artikel dazu!
Jetzt informieren!