|
16.02.2023 22:56:00
|
Final Long-Term Results on Landmark Clinical Study Showing Modified Citrus Pectin Successfully Treats Biochemically Relapsed Prostate Cancer
SANTA ROSA, Calif., Feb. 16, 2023 /PRNewswire/ -- Better Health Publishing — Results from an 18 month phase II clinical trial on the benefits of dietary supplement modified citrus pectin in biochemically relapsed prostate cancer will be presented at the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium, Feb 16-18. Results showed that 18 months of treatment with researched modified citrus pectin (P-MCP) reduced prostate cancer progression, stabilized or decreased PSA, and slowed PSA doubling time (PSADT) without hormonal interference or side effects.
This multi-center study enrolled 59 prostate cancer patients from leading oncology centers in Israel. Subjects had increasing PSA for 6 months prior to enrollment, and took 4.8 grams of P-MCP 3 times/day for 6 months. After 6 months, 46 subjects (78%) showed response to P-MCP, with decreased or stabilized PSA in 34 subjects (58%). 44 subjects (75%) showed improvement of PSADT with negative scans. These 6 month results were published in Nutrients.
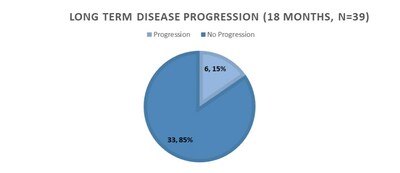
46 of 59 subjects who participated in the first phase were eligible for an additional 12 month treatment phase, totaling 18 months. 7 subjects dropped from the study and purchased P-MCP independently, with continued benefits. The remaining 39 subjects continued for an additional 12 months. The final 18 month results demonstrated 90% of the subjects had improvement or stabilization of PSADT, 62% had decreased or stable PSA, and 85% had no disease progression, biochemically and in scans.
These results highlight the clinically relevant benefits of P-MCP in prostate cancer, offering an evidence-based alternative to hormonal interventions for a population with limited options.
"These results continue to demonstrate P-MCP is safe and effective in biochemically relapsed prostate cancer. Additional clinical research is planned," says Dr. Daniel Keizman, principal investigator.
With 80+ published studies, P-MCP is an extensively researched supplement with previous clinical studies in prostate cancer. This landmark study adds to the fast-growing body of scientific literature on P-MCP in oncology, cardiovascular and kidney health, and other areas.
Dr. Isaac Eliaz, last author states, "These results highlight the importance of safe, non-hormonal alternatives that can halt cancer. This breakthrough study demonstrates P-MCP is in a class of its own as a clinically-proven, non-hormonal adjunct with substantial benefits in prostate cancer, and other areas."
Sources:
Keizman D, et al. Nutrients. 2021; 13(12):4295.
Abstract poster at ASCO GU Cancer Symposium 2023
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/final-long-term-results-on-landmark-clinical-study-showing-modified-citrus-pectin-successfully-treats-biochemically-relapsed-prostate-cancer-301749379.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/final-long-term-results-on-landmark-clinical-study-showing-modified-citrus-pectin-successfully-treats-biochemically-relapsed-prostate-cancer-301749379.html
SOURCE Better Health Publishing
 Der finanzen.at Ratgeber für Aktien!
Der finanzen.at Ratgeber für Aktien!
Wenn Sie mehr über das Thema Aktien erfahren wollen, finden Sie in unserem Ratgeber viele interessante Artikel dazu!
Jetzt informieren!