|
29.09.2022 22:30:00
|
Vanda Pharmaceuticals and OliPass Announce Strategic Partnership to Develop Antisense Oligonucleotide Therapeutics
WASHINGTON, Sept. 29, 2022 /PRNewswire/ -- Vanda Pharmaceuticals Inc. (Vanda) (Nasdaq: VNDA) and OliPass Corporation (OliPass) (KOSDAQ: 24460) today announced that they have entered into a research and development collaboration agreement to jointly develop a set of antisense oligonucleotide (ASO) molecules based on OliPass' proprietary modified peptide nucleic acids. This innovative partnership leverages the respective strengths of Vanda and OliPass to support the development of ASO-based precision medicine therapeutics and potentially create compelling value opportunities for both companies.
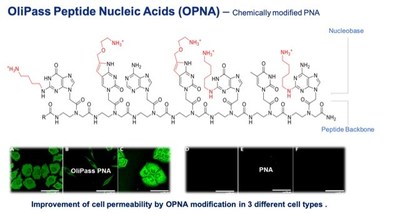
The collaboration will focus on editing and modifying gene expression using ASOs in disease states where the expression of genes is either altered or the sequence of the expressed genes can be altered for therapeutic benefit. OliPass' unique Olipass Peptide Nucleic Acids (OPNA) technology provides the delivery platform to enable these gene expression modifications.
"We are excited to have a partner to enhance our antisense oligonucleotide program platform aimed at the treatment of disorders with well-understood genetic mechanisms and for which there is a high unmet medical need," said Mihael H. Polymeropoulos, M.D., Vanda's President, CEO and Chairman of the Board. "Olipass' delivery platform holds the promise of efficient delivery of our ASO's in pursuit of the development of precision therapeutics."
"Vanda shares a vision with us on the potential for anti-sense oligonucleotides to open up a broad range of therapeutic options to patients," said Dr. Shin Chung, CEO of OliPass. "We look forward to working together to enhance the therapeutic profile of these molecules and playing an integral role in bringing them into the clinical setting."
Vanda has already identified two ASO targets that have been validated in cell lines that model two undisclosed disease targets, one rare orphan and the other applicable to a broad set of immuno-oncological conditions. Vanda's partnership with OliPass to enhance the existing ASOs with OliPass' unique OPNA chemistry is the next step to take these preclinical findings to in vivo and clinical testing.
OPNAs are selectively modified with cationic moieties that enhance both stability and cell permeability of peptide nucleic acids (PNA) while maintaining very high binding affinities to target nucleic acids. Therapeutic efficacy for OPNAs has been observed with dosages as low as 10 ng/kg in animal models, showing effects at dosages many orders of magnitude lower than previously achievable with existing ASO technologies. Furthermore, OPNAs have the capacity to bind to pre-mRNA in nucleus and enable targeting pathogenic variants and conditions that were previously untreatable.
About Vanda Pharmaceuticals Inc.
Vanda is a leading global biopharmaceutical company focused on the development and commercialization of innovative therapies to address high unmet medical needs and improve the lives of patients. For more on Vanda Pharmaceuticals Inc., please visit www.vandapharma.com and follow us on Twitter @vandapharma.
About OliPass
OliPass Corporation is a public biotech company listed in KOSDAQ in South Korea. The company is developing RNA therapeutics based on its proprietary oligonucleotide platform called OPNA (OliPass Peptide Nucleic Acids). OPNA was derived from PNA by rational chemical modifications in order to improve the cell permeability and RNA affinity. For therapeutic intervention, OPNA potently binds to target pre-mRNA, induces exon skipping, and yields mRNA splice variant. Unlike other types of RNA therapeutics, OPNA does not require formulational aid for in vivo therapeutic activity.
CAUTIONARY NOTE REGARDING FORWARD LOOKING STATEMENTS
Various statements in this press release, including, but not limited to, statements regarding the proposed research and development activities of the strategic partnership between Vanda and OliPass, and the potential commercial and therapeutic opportunities that may result from it, are "forward-looking statements" under the securities laws. All statements other than statements of historical fact are statements that could be deemed forward-looking statements. Forward-looking statements are based upon current expectations and assumptions that involve risks, changes in circumstances and uncertainties. Important factors that could cause actual results to differ materially from those reflected in such forward-looking statements include, among others, the ability of Vanda and OliPass to leverage their expertise to successfully edit and modify gene expression using ASOs, the ability of OliPass' platform to efficiently deliver Vanda's ASO's into cells, Vanda's ability to progress the two ASO targets it has identified from pre-clinical to clinical testing, and Vanda's ability to complete the development of and obtain regulatory for these ASO targets. Therefore, no assurance can be given that the results or developments anticipated by Vanda will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, Vanda. Forward-looking statements in this press release should be evaluated together with the various risks and uncertainties that affect Vanda's business and market, particularly those identified in the "Cautionary Note Regarding Forward-Looking Statements", "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" sections of Vanda's Annual Report on Form 10-K for the fiscal year ended December 31, 2021, as updated by Vanda's subsequent Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and other filings with the U.S. Securities and Exchange Commission, which are available at www.sec.gov.
All written and verbal forward-looking statements attributable to Vanda or any person acting on its behalf are expressly qualified in their entirety by the cautionary statements contained or referred to herein. Vanda cautions investors not to rely too heavily on the forward-looking statements Vanda makes or that are made on its behalf. The information in this press release is provided only as of the date of this press release, and Vanda undertakes no obligation, and specifically declines any obligation, to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
Vanda Corporate Contact:
Kevin Moran
Senior Vice President, Chief Financial Officer and Treasurer
Vanda Pharmaceuticals Inc.
202-734-3400
pr@vandapharma.com
Elizabeth Van Every
Head of Corporate Affairs
Vanda Pharmaceuticals Inc.
202-734-3400
pr@vandapharma.com
OliPass Corporate Contact:
Jason Kim
Director, Finance
OliPass Corporation
+82(0)2 6488 2232
jkim@olipass.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/vanda-pharmaceuticals-and-olipass-announce-strategic-partnership-to-develop-antisense-oligonucleotide-therapeutics-301637296.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/vanda-pharmaceuticals-and-olipass-announce-strategic-partnership-to-develop-antisense-oligonucleotide-therapeutics-301637296.html
SOURCE Vanda Pharmaceuticals Inc.
 Der finanzen.at Ratgeber für Aktien!
Der finanzen.at Ratgeber für Aktien!
Wenn Sie mehr über das Thema Aktien erfahren wollen, finden Sie in unserem Ratgeber viele interessante Artikel dazu!
Jetzt informieren!
Nachrichten zu Vanda Pharmaceuticals IncShsmehr Nachrichten
|
30.07.24 |
Ausblick: Vanda Pharmaceuticals öffnet die Bücher zum abgelaufenen Quartal (finanzen.net) |